Reading time: 6 minutes

Datei:Strontianit_und_Schwefel-1697.jpg
THINGS TO KNOW Today, sulphuric acid is considered one of the most important chemicals of all. Due to its properties, the substance is used in numerous branches of industry. It can be produced on a large scale from sulphur by the double contact process.
The term sulphuric acid (H2SO4) stands for a strongly corrosive, inorganic acid that is colourless, oily, viscous and hygroscopic (dehydrating) in its undiluted liquid state. It is an important basic chemical and is used to produce numerous other substances (cf. Table 1). The main quantity (approx. 50 – 60% worldwide) is used for the production of fertilisers such as superphosphate.

The acid is also necessary for the production of dyes by ore digestion, an example would be the white pigment titanium dioxide, which is produced from ilmenite (FeTi03) in several steps. Furthermore, the substance together with nitric acid is a component of nitrating acid, which is used for the production of nitro compounds, for example for the production of explosives. Diluted sulphuric acid is also used as an electrolyte in lead accumulators. Concentrated sulphuric acid, on the other hand, is a very good drying agent due to its hygroscopic properties.
The double contact process
In the past, sulphuric acid was produced using the vitriol process (from the 13th century) and the lead chamber process (from the 18th century). A modern large-scale process for the production of sulphuric acid was developed by the German chemists Clemens Winkler and Rudolf Knietsch in the 19th century. However, this so-called “contact process” is already obsolete and has been replaced by the more efficient and environmentally friendly double contact process. The latter is used today and can basically be divided into three steps. The double contact process runs according to a continuous mode of operation, i.e. starting materials are fed in (almost) continuously and reaction products are discharged.
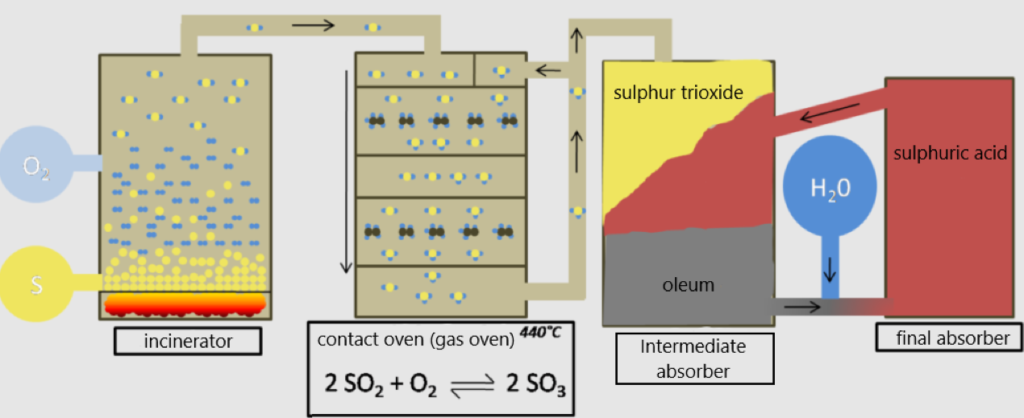
Source: https://commons.wikimedia.org/wiki/File:Doppelkontaktverfahren2.png
1 Incinerator
For the technical synthesis of sulphuric acid (the first step of the double contact process), sulphur trioxide is required. For this purpose, sulphur dioxide is first obtained by burning liquid sulphur (an initial temperature of 135°C is suitable) with atmospheric oxygen in the combustion furnace with the aid of a burner. The oxidation that takes place is strongly exothermic: S + 02 -> SO2.
2 Contact oven

Source: https://commons.wikimedia.org/wiki/
File:La_Sorbonne._M._le_professeur_
H._Le_Chatelier,_membre_de_l%27Institute.jpg
This mixture is cooled down to 420°C until it is further converted (oxidised) to sulphur trioxide in the so-called contact oven, another exothermic reaction: 2 SO2 + O2 -> 2 SO3. For this to take place, the sulphur dioxide is passed through several sieve trays, so-called trays, with a heap of the catalyst vanadium(V) oxide (V2O5).
For an ideal yield of sulphur trioxide and greater efiiciency of the double contact process, the following reaction conditions must apply:
- Temperature: The reaction temperature should be 420 – 440°C. With higher temperature the amount of sulphur trioxide would decrease (exothermic chemical equilibrium – principle of Le Chatelier), but even lower temperatures would slow down the reaction rate (working temperature catalyst above 400°C). In order to maintain this operating temperature, the excess energy generated in the hordes is dissipated through heat exchangers (past the cold starting materials according to the countercurrent principle).
- Pressure: The substances react under normal pressure, as the GG constant has a comparatively high and thus favourable value (66 kPa-1) at around 450°C (see Le Chatelier’s principle under a reaction with volume decrease).
- Concentration changes: Similarly, one can increase the use of oxygen because more starting materials mean more reaction products. If some of the sulphur trioxide produced is discharged, more reaction products are also produced.
3 Absorber
The sulphur trioxide is cooled to approx. 70°C and fed into absorber towers, where it first reacts with concentrated sulphuric acid (98%) to form oleum. This so-called “fuming sulphuric acid” is a mixture of sulphuric acid, dissolved sulphur trioxide and disulphuric acid (H2S207). Under cooling, a certain amount of water is then added to this oleum (determination of the water content via conductivity measurements), so that sulphuric acid is formed:
H2SO4 + SO3 -> H2S2O7 and H2S2O7 + H20 -> 2 H2SO4.
Since not all of the sulphur dioxide is converted in the first pass, the remaining amount is fed into a contact furnace again until at least 99.5% sulphuric acid is produced in the final absorber (hence the name “double contact process”). The residual gas is released into the atmosphere.
By the way: Learning about the double contact process is not enough for you? You are also interested in the production of calcium cyanamide and its importance for the city of Wittenberg? Click here.
Bibliography
Arnold, Karin/Dietrich, Volkmar u.a., Cornelsen Verlag (Hrsg.). (2009). Chemie Oberstufe, Allgemeine Chemie, Physikalische Chemie, Berlin: Cornelsen Verlag, S. 138 ff.
Lernhelfer: Verwendung von Schwefelsäure. Access on 04/07/2020
Spektrum.de: Schwefelsäure. Access on 04/07/2020
Seilnacht.com: Doppelkontaktverfahren. Access on 04/07/2020
Schule-Studium.de: Großtechnische Verfahren: Das Doppelkontaktverfahren zur Herstellung von Schwefelsäure. Access on 04/07/2020
Fokus-Online: Schwefelsäure: Eigenschaften und Verwendung der Chemikalie. Access on 04/07/2020
Chemie.de: Schwefelsäure. Access on 04/07/2020
Lernhelfer: Großtechnische Herstellung von Schwefelsäure. Access on 11/07/2020

